Organic Light Emitting Diodes
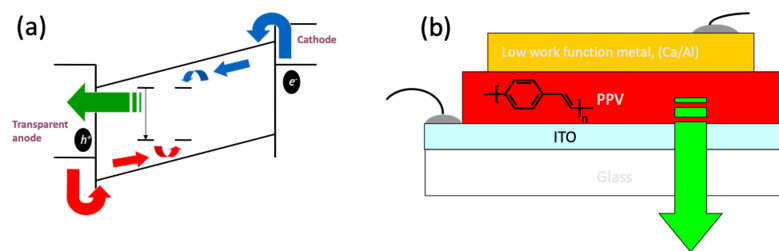
Organic Light Emitting Diodes (OLED) convert electrical current into light. A typical OLED device consists of several electrical and photoactive materials sandwiched between two electrodes. Figure 1(a) depicts a schematic operating OLED device. The light emission process is based on the recombination of electron injected from the cathode with holes injected from the transparent anode in this case. In general this process is similar to the one taking place in inorganic LED. The difference is in the processes that take place within the material/materials sandwich between the electrodes.
Figure 1. Schematic structure of an OLED showing electrical energy convert to emitted light (a). An example of such a simple structure for green light emission. After J. H, Burroughes et. al. Nature, 347, 539 (1990)
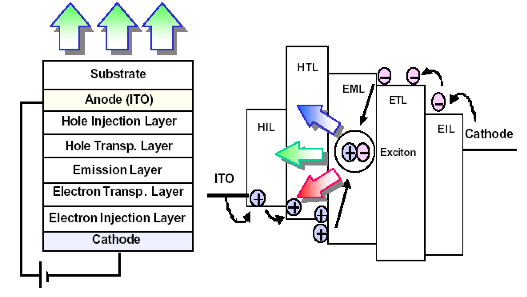
Such a simple structure as showed in Fig. 1 (b) yield a very low efficiency. The reason is that the emitted light is due to a recombination of an exciton, a bounded electron-hole pair, and not a direct recombination of electron in the conduction band and a hole in the valance band. The position of exciton formation need to be controlled because in principle an exciton is a dipole and therefore can be quenched or recombine non-radiatively if it is formed closed to a metallic surface. To achieve this goal a more complex structure that enables control over charge injection, charge transport and the exact position of exciton formation is shown in Fig 2.
Figure 2. An optimized OLED structure consisted of charge injection layers (EIL &HIL), charge transport layers (ETL & HTL) for electrons and holes, respectively. The excitons are formed in a designated emissive layer (EML).
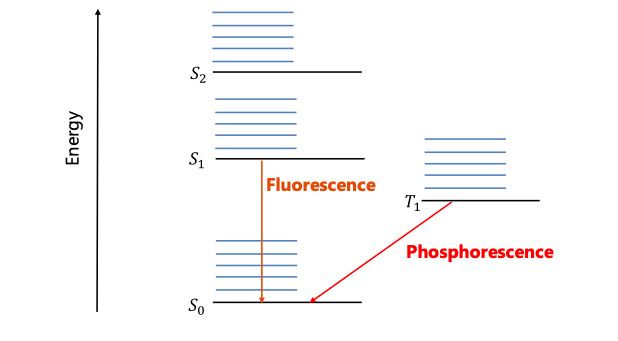
Another efficiency limiting factor is singlet vs. triplet recombination which are usually referred to as fluorescence vs phosphorescence. The exciton is a quantum mechanical specie that is consistent of two fermions. Therefore, it can be either in a triplet or in a singlet state. In organic molecules the only allowed recombination processes is singlet recombination (fluorescence) while triplet recombination is forbidden. This leads to maximum 25% efficiency. However, by adding heavy metal atoms and taking advantage of the spin-orbit coupling process the triplet states are also allowed to recombine (phosphorescence) leading to very high yield as shown in Fig. 3.
Figure 3. Singlet recombination (fluorescence) vs triplet recombination (phosphorescence) processes.